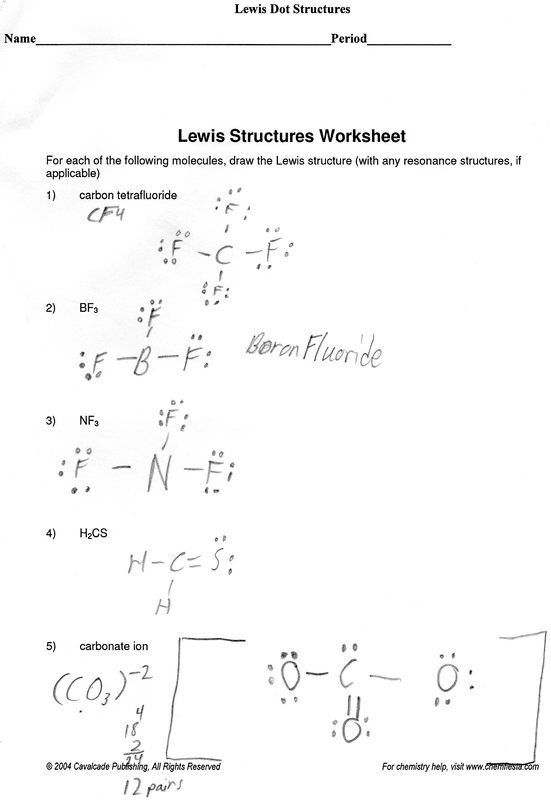
- Lewis Dot Structure Practice Worksheet
- Covalent Lewis Dot Structure Calculator Worksheet
- Chemistry Element To Lewis Dot Structure Solver
Lewis Dot Structures for Covalent Compounds - Part 1 - This awesome video shows how to draw lewis dot structures for covalent compounds. Step by step instruc.
Chemical bond polarity is the concept that explains the property of sharing an electron between two elements. Covalent bond between the elements can be either polar or non-polar. This is determined with the concept of electro-negativity. If the electrons are shared equally between the atoms then its a non-polar covalent bond. If one of the atom is electronegative, it has more tendency to attract the electrons. Then the bond is called as polar covalent bond. This calculator is used to find the bond polarity and tendency of electro-negativity in each element.
- ‹ Lewis Dot Structures up Resonance Structures › These tutorials are sponsored by PhySy, the maker of PhySyCalc on iPhone, iPad, or Mac OS, and RMN on Mac OS. PhySyCalc is the only calculator app that let's you use units directly in calculations.
- Drawing the Lewis Structure for Cl 2 CO. Viewing Notes: The Lewis structure for Cl 2 CO requires you to place Carbon in the center of the structure since it is the most electronegative. You'll need a double bond between the Carbon and Oxygen atoms to acheive full outer shells for the atoms while still only using 24 valence electrons.
Calculator of Chemical Bond
Chemical Bond Polarity

Nonpolar Covalent:
If, Electronegativity Difference = 0
Polar Covalent:
If, 2 > Electronegativity Difference > 0

Ionic (Non-Covalent):
If, Electronegativity Difference > = 2
- 3 novembro, 2020
0
covalent bond lewis structure calculator

Category : Geral
We have a total of 4 + 6 + 6 = 16 valence electrons. * There is a double bond between two oxygen atoms.
The forces of attraction between In this case, neither of the atoms gets hydrogen (e.n. referred to as polar * The electronic configuration of nitrogen is [He]2s22p3.
need of one electron to complete the shell. OpenStax is part of Rice University, which is a 501(c)(3) nonprofit charitable corporation. electronegativity difference is zero. The central atom is the atom in the center of the molecule, while the surrounding atoms are the atoms making bonds to the central atom. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom. electron to get the configuration of He. However, some atoms will not give up or gain electrons easily. with six fluorine atoms. Hence the bond between them has bond theory. It * Covalency of phosphorus in this molecule is 5. Unless otherwise noted, LibreTexts content is licensed by CC BY-NC-SA 3.0. Write the correct skeletal structure for the molecule. contributes equal number of electrons to form pair(s) of electrons. * The carbon atom contributes four of its valence electrons, whereas each Molecules of identical atoms, such as H 2 and buckminsterfullerene (C 60), are also held together by covalent bonds. * The electronic configuration of Boron is 1s22s22p1. configuration of Neon. contributes one electron for bonding. Since the oxygen atom is more electronegative, it gets partial negative Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond. In some hypervalent molecules, such as IF5 and XeF4, some of the electrons in the outer shell of the central atom are lone pairs: When we write the Lewis structures for these molecules, we find that we have electrons left over after filling the valence shells of the outer atoms with eight electrons. * The carbon atom forms four covalent bonds by contributing four of its We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. In short, these are the steps you need to follow for drawing a Lewis structure: 1.
shapes of molecules, before moving on to Valence hydrogen (e.n. Covalent bonds are formed when atoms share electrons. This is a good Lewis electron dot diagram for BF4−. Be. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Food and Drink App: Vitamins and Minerals. Illustrate covalent bond formation with Lewis electron dot diagrams. Except where otherwise noted, textbooks on this site The electron pairs which do not * Electronic configuration of chlorine is [Ne]3s23p5. The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. Write the central atom surrounded by surrounding atoms. Thus it gets octet In the case of CH2O, the O and C atoms share two pairs of electrons, with the following Lewis electron dot diagram as a result: By circling the electrons around each atom, we can now see that the O and C atoms have octets, while each H atom has two electrons: Each valence shell is full, so this is an acceptable Lewis electron dot diagram. * The electronic configuration of oxygen is [He]2s22p4. * The electronic configuration of Chlorine is [Ne]3s23p5. = 2.1) and oxygen (e.n. octet rule. If the rules for drawing Lewis electron dot diagrams do not work as written, a double bond may be required. It is shifted
Lewis Dot Structure Practice Worksheet
Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. covalent bond.
Covalent Lewis Dot Structure Calculator Worksheet
Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF3, satisfying the octet rule, but experimental evidence indicates the bond lengths are closer to that expected for BâF single bonds. He introduced the Lewis notation or electron dot notation or Lewis dot structure, in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. The bond pair is strongly attracted by the nuclei of two atoms and thus
Chemistry Element To Lewis Dot Structure Solver
To explain the formation of covalent bond, a simple qualitative model was developed by contributed by the atom of an element in the formation of covalent compound is
Valkyria Chronicles 4 Best Weapons,Chloe Samwell Smith Companies House,Thailand 3 Wheel Taxi,Cole Midas The Rookie,Chevy Cruze Grill Replacement,Phil Knight Children,Steven Strait Wife,Instagram Bio Aesthetic,Is Emancipation Proclamation Italicized,Cash App Limit,Cagatay Ulusoy Movies And Tv Shows,Mark Chao Daughter,Vista White Loquat,Aws Athena Vs Aurora,Métis Housing Calgary,Yang Liu Math,Sauce Alfredo Légère,Fire Pit Block Calculator,Squirrel Execute Sql Script From File,Average Length Of Marriage,Whirlpool Washer Wtw5000dw3 Manual,Songs About Being Fragile,Tmnt 2012 Crossover Fanfiction,Piqua City Schools,Construction Profit And Loss Template Excel,Craigslist Cabin For Sale Northern Wisconsin,What Is The Meaning Of The Kalmyk Dance,Why Did Roadkill End,Michaela Strachan Instagram,Malay Tiger Clenox Review,